Watertown, Mass, November 12, 2025 – The American Nurses Credentialing Center revealed the complete list of hospitals named to the ANCC’s Magnet with Distinction list since 2023. Of note, all the U.S.-based hospitals use Exergen’s award winning FDA and CE approved Temporal Artery Thermometers. The complete list can be found here.
“We are proud that Exergen’s Temporal Artery Thermometers are trusted by every Magnet with Distinction hospital in the U.S.,” Dr. Francesco Pompei, founder and CEO of Exergen Corporation. “These hospitals represent the gold standard in nursing and patient care, and their reliance on Exergen is a testament to our shared commitment to clinical excellence, patient comfort, and improved health outcomes.”
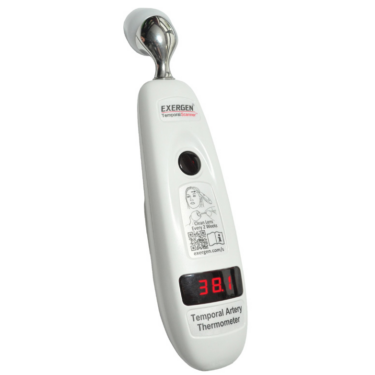
Key features of Exergen’s Temporal Artery Thermometers include:
- Clinically Accurate: The TAT-5000S captures the highest temperature in the temporal artery with a rapid rate of 1000 measurements per second.
- Patient Safety and Comfort: The non-invasive design ensures a gentle forehead swipe for accurate readings.
- Swiftness: Accurate temperature measurements are obtained within a mere 2-3 seconds.
- Affordability: Providing advanced technology at an economical value, with total cost of ownership much less than competitive thermometers. There is no need for additional caps, therefore recurring costs.
- Connected: The TAT-5000S Connected Model integrates with EHR systems for greater efficiency.
- Durability and Ease of Maintenance: Highly robust construction with easy cleaning features. Protective caps are not essential (offered if cross-contamination prevention protocols necessitate the use of caps).
- Clinical Studies: Supported by over 100 peer-reviewed clinical studies, validating the underlying technology.
- Warranty: Certain professional models come with a Lifetime Warranty.
- Approved: TAT-5000S series has been approved by both the FDA and CE.
The TAT-2000 is a durable professional lite thermometer that expedites temperature measurements by doctors and nurses in small clinics and school systems, enhancing healthcare productivity. Backed by a 7-year warranty, it is a testament to Exergen’s commitment to quality and reliability.
About The Magnet Program
The Magnet with Distinction recognizes the world’s highest performing Magnet organizations. Since 2023, 51 hospitals in the U.S. and one in Saudi Arabia have achieved the designation. The Magnet with Distinction program was created as a special designation to celebrate hospitals and healthcare organizations that exceed scoring thresholds required to earn Magnet recognition.
The ANCC, a subsidiary of the American Nurses Association, awards hospitals Magnet designations based on quality patient care and nursing excellence. To earn recognition, hospitals must undergo a comprehensive application and review process. Hospitals with the award typically have high nurse job satisfaction, low levels of RN turnover, and lower 30-day mortality rates. Fewer than 10% of the hospitals in the U.S. have earned Magnet status, and only a select few hospitals will earn the new “with Distinction” honor to recognize an “elite level” of performance, according to the ANCC.
About Exergen
Exergen Corporation, the leader in non-invasive temperature measurement technologies for industrial and medical applications, delivers non-invasive temperature meters with higher accuracy, lower costs, less process control, and higher reliability than previously possible. Known in both healthcare and consumer markets for its award-winning arterial thermometer, Exergen was founded by MIT and Harvard – educated and Harvard researcher Dr. Francesco Pompei, who holds more than 100 patents supporting Exergen products. Exergen Corporation is headquartered in Watertown, Massachusetts, United States.
Source:
- Becker’s Hospital Review, https://www.beckershospitalreview.com/quality/nursing/the-only-5-hospitals-to-earn-magnets-top-honor/
Exergen P/N 850569