Exergen Praises FDA for Warning of Limitations of Use of Non Contact Infrared Thermometers (NCITs) Due to Potential Unreliability
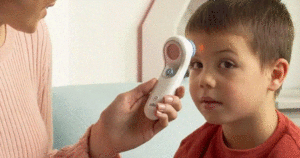 The FDA provided an update that factors such as measurement location, distance of the sensor, individual physiological attributes and environmental conditions can impact the reliable performance of NCITs.
The FDA provided an update that factors such as measurement location, distance of the sensor, individual physiological attributes and environmental conditions can impact the reliable performance of NCITs.
WATERTOWN, Mass., Nov.13, 2023 – Exergen, a leading manufacturer of electronic thermometers and maker of the most accurate thermometer on the market, today applauds the FDA for updating its webpage warning the public of the limitations of non‑contact infrared thermometers (NCITs).[1] During the pandemic, NCITs became a popular tool for public temperature screenings even though clinical research has proven that they often fail to reliably detect fevers.
The FDA’s updated webpage underscores the limitations of NCITs, noting that while they can provide accurate and reliable body temperature measurements in specific settings and conditions, there are a wide number of factors that impact the performance of such devices including measurement location, distance of the sensor, individual physiological attributes and environmental conditions..
In contrast, the performance and accuracy of the Exergen TemporalScanner is supported by 100 peer-reviewed published clinical studies. Additionally, a recent Newsweek article listed the top U.S. hospitals in each state. All of these hospitals use the Exergen Temporal Scanner to provide high quality patient care. Exergen is committed to helping protect public health by providing only medically accurate thermometers for retail sale as opposed to NCITs.
“The FDA’s recognition of the limitations of NCITs and their potential lack of reliability is an important stake in the ground that consumers and all those passionate about public health should be rejoicing about,” said Francesco Pompei, Ph.D., CEO of Exergen Corporation. “Since body temperature is such a critical health indicator, we urge consumers, retailers and health professionals alike to consider the negative implications that the use of NCITs can have on public health and opt for a more accurate and clinically proven option like the Exergen TemporalScanner. When it comes to taking a person’s temperature to detect illness, nothing is more important than accuracy and reliability, and that has been our passion for decades – and why our technology is used not only by millions of families throughout the country, but also by the nation’s top doctors, nurses, hospitals, pediatric centers and medical institutions.”
The Exergen TemporalScanner easily and accurately takes temperature measurements of the temporal artery. Using patented Arterian Heat Balance system technology, Exergen ensures temperature readings are accurate by mathematically accounting for ambient temperatures and the cooling effect of the scanner. By capturing naturally emitted heat over the temporal artery, Exergen makes it easy to get an accurate body temperature with a quick scan of the forehead.
For additional information or to purchase an Exergen thermometer, visit www.exergen.com.
About Exergen Corporation
Exergen invented, manufactures, and markets two series of the TemporalScanner thermometer: a professional version for hospitals and clinics, and a consumer version sold in major retailers nationwide. More than three billion temperatures are taken each year with TemporalScanners. Used in thousands of hospitals and clinics across the country as well as in millions of homes, TemporalScanners are the #1 preference of pediatricians, nurses, and parents. The ExergenTemporalScanner's accuracy is supported by more than 100 peer-reviewed published clinical studies covering all ages from preterm infants to geriatrics and all care areas from hospitals to homes. Exergen has also long been a leader in nursing. For nearly 20 years, it has been part of the nursing profession's educational curriculum. Published textbooks from 2005 to present include Exergen thermometers and have set nurse training standards, relied upon in thousands of nursing programs nationwide.
Press Contact:
[1] See https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/non-contact-infrared-thermometers.
PN 850115
